Active Ingredient Prescribing (AIP) is an initiative announced by the Australian Government in the 2018-19 budget, with the purpose of promoting better patient understanding of the medications they are taking, and encouraging the use of generic medicines. Active Ingredient Prescribing will become mandatory as of 1 February, 2021, and is included in the Orchid SP1 Revision 1 edition of Bp Premier.
When your practice upgrades to Orchid SP1 Revision 1, the Active Ingredient Prescribing related changes in this release will mean that prescriptions written for PBS and RPBS medication will have the medication's active ingredients listed on them by default, rather than a brand name.
Active Ingredient Prescribing will have a minimal impact on the existing prescribing work flow in Bp Premier. It will still be possible to select medication by brand name when prescribing, and the option to include a brand name on the script along with the active ingredients is available if required.
For more information on Active Ingredient Prescribing, visit the Australian Government Department of Health and Aged Care website.
See also Frequently Asked Questions about Active Ingredient Prescribing
Warfarin or Clozapine
In a number of Australian states and territories, it is a legislative requirement to include brand name on prescriptions for Warfarin and Clozapine, to support patient safety. Where your practice's jurisdiction mandates inclusion of brand name for these drugs, you must tick the Print Brand Names on Script check box when prescribing.
When prescribing either drug, Best Practice Software recommend the prescriber search in the New Rx screen as follows:
- Search for the drug by brand name, and select the drug to prescribe by brand
- Search for the drug by generic name, and click Equivalent products to select the drug by brand rather than generic.
or
You must tick the Print Brand Names on Script check box when finalising the prescription.
List of Medicines for Brand Consideration (LMBC)
The List of Medicines for Brand Consideration (LMBC) identifies medicines for which you may wish to consider if including the brand name on the prescription is necessary for the patient.
This is a guide intended to support clinical decision making only. The decision as to whether or not a drug is prescribed by brand name or not is still made by the prescribing doctor, based upon the appropriate clinical outcome for the patient.
If you prescribe a drug that is included on the LMBC in Bp Premier, a message will appear in the New Rx wizard, indicating that you may wish to consider prescribing by brand.
View the LMBC on the Australian Government Department of Health and Aged Care website.
List of Excluded Medicinal Items (LEMI)
There are a number of medicines and prescription types that are excluded from Active Ingredient Prescribing. These include:
- handwritten prescriptions
- prescriptions generated through a free text function within prescribing software
- custom preparations
- paper-based medication charts in residential aged care facilities
- over the counter medication
- non-medicinal PBS and RPBS items, such as dressings and food supplements
- prescriptions for medication with four or more active ingredients
- vaccines.
View the LEMI on the Australian Government Department of Health and Aged Care website.
Example prescriptions
Here are some examples of how prescriptions will appear once active ingredient prescribing is introduced:
Prescriptions list the active ingredient followed by dosage strength. If the medication contains two or three active ingredients, all active ingredients are printed, separated by a plus sign.

If you have chosen to list a brand name on the prescription, it will be included in brackets after the active ingredients.

If you have prescribed over the counter medication, non-medicinal PBS and RPBS items, vaccines, or medication with four or more active ingredients, only the brand name will be listed on the prescription.

Prescribing information in other areas of Bp Premier
Areas in Bp Premier that display previews of prescriptions, such as in the prescription lookup feature, will display medications as they would appear on the prescription.
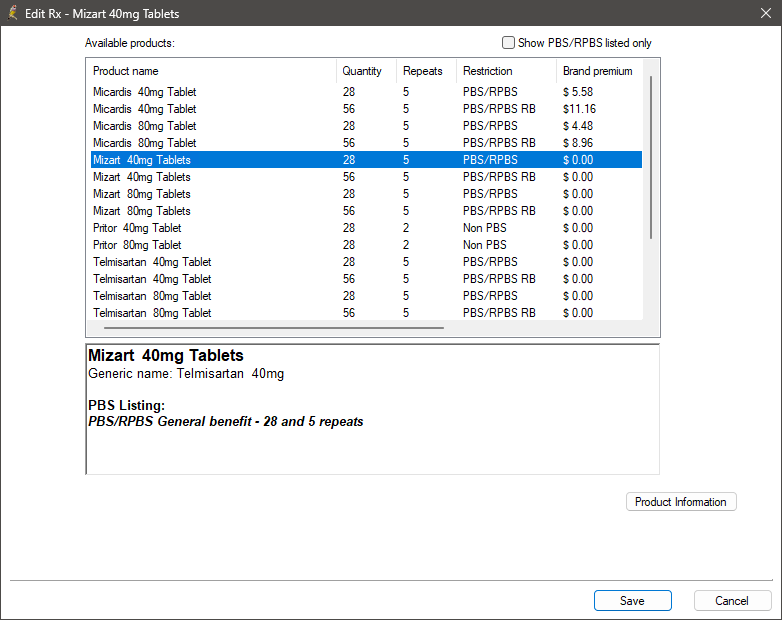
Other areas of Bp Premier that display prescribing information such as the Current Rx screen, Word Processor, or drug sheets, will display prescribing information as per what has been selected through the New Rx wizard. For example, if you select the brand 'Micardis' in the New Rx wizard, 'Micardis' will be listed in the Current Rx screen, while the active ingredient 'Telmisartan' will be listed on the prescription.
Active Ingredient Prescribing and the Allow brand substitution check box
In the prescribing workflow, you can select Print brand names on scripts and Allow brand substitution at the same time if you wish. If so, the pharmacist may dispense the patient a brand equivalent substitution that is biosimilar.
The Allow brand substitution check box functionality is linked to the Print brand name on scripts check box. If you select a brand in the first panel of the New Rx wizard, the Allow brand substitution check box in the third panel will be greyed out and unticked. If you select Print brand name on scripts, the Allow brand substitution check box will be enabled and ticked. You can then choose whether or not to leave the Allow brand substitution check box ticked.
Prescriptions for existing medication
If a patient's medication has been prescribed as not allowing brand substitution in a previous version of Bp Premier, it will be prescribed by brand name as the prescriber has previously indicated that the patient must be on the defined brand of medication.
If brand substitution has been allowed for an existing medication in previous versions of Bp Premier, the medication will not be flagged to be prescribed by brand name, as the prescriber has previously indicated that generic brands can be dispensed.
Re-prescribing discontinued brands
If you attempt to re-prescribe a brand of medication that has been discontinued, an alert will appear indicating that the product is no longer available, and you will have the option to select an alternative brand. You will only be given the option to prescribe alternatives if you attempt to re-prescribe from the Current Rx screen.
If you choose to select an alternative brand, the Change brand screen will appear listing any alternative brands available, as well as the medication's generic name. Select an option from the list, and click Save to prescribe the alternative.
Last updated: 29 November 2023.