What is Active Ingredient Prescribing?
Active Ingredient Prescribing is a new way of listing medication on prescriptions. Rather than listing a brand name on a prescription, the active ingredients in the medication will be listed instead.
Why was Active Ingredient Prescribing introduced?
Active Ingredient Prescribing has been mandated by the government. It was introduced to increase patient understanding of the medication they are taking, and to promote the uptake of generic and biosimilar medication.
This resource from NPS MedicineWise provides more information.
Which version of Bp Premier includes Active Ingredient Prescribing?
Bp Premier Saffron edition.
Is Active Ingredient Prescribing be mandatory?
Yes. Active Ingredient Prescribing has been mandatory since 1 February, 2021.
How does Active Ingredient Prescribing affect the way I prescribe in Bp Premier?
Changes to Bp Premier's prescribing workflow are minimal. When prescribing medication, if you wish to have the brand name printed on the prescription, you must tick a checkbox.
Are there any guidelines around which medications should be prescribed by brand or by active ingredient?
The List of Medicines for Brand Consideration (LMBC) indicates drugs that you may wish to consider prescribing by brand name. The LMBC is a guide only and the decision still lies with the prescribing doctor. Medicines on the LMBC are indicated in the Bp Premier prescribing workflow.
If I need to re-prescribe medication for a patient, will Bp Premier remember if I have previously chosen to tick the checkbox enabling the brand name to display on the prescription?
Yes, Bp Premier will remember this choice for a particular patient and medication.
Do all prescriptions list active ingredients now instead of a brand name?
Most do, but there are some exceptions. These exceptions are:
- handwritten prescriptions
- prescriptions generated through a free text function within prescribing software
- custom preparations
- paper-based medication charts in residential aged care facilities
- over the counter medication
- non-medicinal PBS and RPBS items, such as dressings and food supplements
- prescriptions for medication with four or more active ingredients
- vaccines
In these cases, the brand name is listed on the prescription. See What will prescriptions look like once Active Ingredient Prescribing is introduced? for more information.
Do I have to remember which products are excepted from Active Ingredient Prescribing?
No, if you prescribe an excepted product, the brand name will be listed on the prescription automatically.
Can I change the default prescription settings to prescribe medication by brand name instead of active ingredients?
No. While you can choose to prescribe medication by brand name if you wish, you need to choose this option on a per prescription basis through the New Rx wizard. There is no option to set prescribing by brand name as the default through Setup > Preferences > Prescribing.
How does medication appear on drug sheets?
Medication on drug sheets will list the brand name and dosage, followed by the active ingredients in brackets.
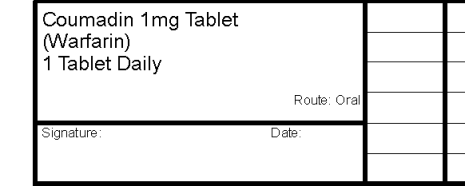
Is it still possible to print a brand name on the script if I want?
Yes, there is an option to print the brand name on a prescription, although the default option will be to print the active ingredients only. If the brand name is included, it will appear in brackets after the active ingredients.
What will prescriptions look like once Active Ingredient Prescribing is introduced?
Prescriptions list the active ingredient followed by dosage strength. If the medication contains two or three active ingredients, all active ingredients are printed, separated by a plus sign.

If you have chosen to list a brand name on the prescription, it will be included in brackets after the active ingredients.

If you have prescribed over the counter medication, non-medicinal PBS and RPBS items, vaccines, or medication with four or more active ingredients, only the brand name will be listed on the prescription.

Last updated 01 February 2021